Publications (*corresponding author) Google Scholar citations
- Lopez-Nieves S., Yang Y., Timoneda T., Wang M., Feng T., Smith S.A., Brockington S.F., Maeda H.A.* (2017) Relaxation of Tyrosine Pathway Regulation Underlies the Evolution of Betalain Pigmentation in Caryophyllales. Accepted to New Phytol.
- Schenck C.A., Holland C.K., Schneider M., Men Y., Lee S.G., Jez J. and Maeda H.A.* (2017) Molecular Basis of the Evolution of Alternative Tyrosine Biosynthetic Routes in Plants. Nature Chem. Biol. DOI:10.1038/nchembio.2414 Also, read UW News. Free view-only version
- Wang M. and Maeda H.A.* (2017) Aromatic Amino Acid Aminotransferases in Plants. Phytochemistry Reviews, DOI:10.1007/s11101-017-9520-6. Free view-only version
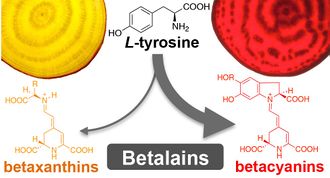
- Wang M., Lopez-Nieves S., Goldman I., Maeda H.A.* (2017) Limited Tyrosine Utilization Explains Lower Betalain Contents in Yellow than Red Table Beet Genotypes. J Agric Food Chem. 65, 4305–4313.
- Betalains are red and yellow alkaloid pigments derived from tyrosine. Previous studies showed that yellow pigments accumulate much less than red pigments. Through quantitative chemical analyses of different beet genotypes, this study found that increased tyrosine levels were positively correlated with elevated betalain accumulations among red but not yellow genotypes, suggesting that yellow beets are not efficiently converting tyrosine into betalain pigments. Thus, better utilization of the accumulated tyrosine can likely improve betaxanthin production in yellow beets.
- Wang M., Toda K., Maeda H.A.* (2016) Biochemical Properties and Subcellular Localization of Tyrosine Aminotransferases from Arabidopsis thaliana. Phytochemistry, 132, 16–25
- Maeda H.A.* (2016) Lignin biosynthesis: Tyrosine shortcut in grasses. Nature Plants 2, 16080 DOI: 10.1038/NPLANTS.2016.80
- Schenck C.A., Chen S., Siehl D., Maeda H.A.* (2015) Non-plastidic, Tyrosine-Insensitive Prephenate Dehydrogenases from Legumes. Nature Chem. Biol. 11, 52-57 *Featured on the Cover.
- L-Tyrosine (Tyr) and its plant-derived natural products are essential in both plants and humans. In plants, Tyr is generally assumed to be synthesized in the plastids via arogenate dehydrogenase (ADH) that is strictly inhibited by Tyr. Here we identified non-plastidic Tyr-insensitive prephenate dehydrogenases (PDHs) uniquely present in legumes, providing molecular evidence for the diversification of primary metabolic Tyr pathway via an alternative cytosolic PDH pathway in plants. Also, the Tyr-insensitive property of the identified PDH enzymes has immediate impacts on metabolic engineering to improve the production of Tyr and Tyr-derived natural products.
- Dornfeld C., Weisberg A.J., Ritesh KC, Dudareva N., Jelesko J.G., Maeda H.A.* (2014) Phylobiochemical Characterization of Class-Ib Aspartate/Prephenate Aminotransferases Reveals Evolution of the Plant Arogenate Phenylalanine Pathway Plant Cell 26, 3101-3114
- Plants use phenylalanine to produce abundant and diverse phenylpropanoid compounds, such as flavonoids, tannins, and lignin. Through phylogenetic, bioinformatic, and biochemical analyses of prephenate aminotransferase enzymes from plant and bacterial lineages, this study revealed unique evolutionary history and molecular changes of key enzymes responsible for phenylalanine biosynthesis in plants. The findings assist the rational design of antimicrobial drugs and herbicides, but also highlight the use of phylobiochemical characterization of enzymes from deep taxonomic lineages in determining key molecular changes that lead to the evolution of new metabolic pathways. UW news release.
- Luby C., Maeda H.A., Goldman I. (2014) Genetic and Phenological Variation of Tocochromanol (Vitamin E) Content in Wild (Daucus carota L. var. carota) and Domesticated Carrot (D. carota L. var. sativa) Horticulture Research 1:15
- Maeda H., Song W., Sage T.L., DellaPenna D. (2014) Role of Callose Synthases in Transfer Cell Wall Development in Tocopherol Deficient Arabidopsis Mutants. Front. Plant Sci. 5:46.
- Yoo H., Widhalma J.R., Qiana Y., Maeda H., Cooperc B.R., Jannaschc A.S., Gondae I., Lewinsohne E., Rhodes D., Dudareva D. (2013) An Alternative Pathway Contributes to Phenylalanine Biosynthesis in Plants via a Cytosolic Tyrosine:Phenylpyruvate Aminotransferase. Nature Commun. 4:2833
- Maeda H. and Dudareva N. (2012) The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Ann. Rev. Plant Biol. Vol. 63, 73-105
- Muhlemann J.K., Maeda H., Chang C.Y., San Miguel P., Baxter I., Cooper B., Perera M.A., Nikolau B.J., Vitek O., Morgan J.A., Dudareva N. (2012) Developmental Changes in the Metabolic Network of Snapdragon Flowers. PLoS ONE 7(7): e40381
- Maeda H., Yoo H., and Dudareva N. (2011) Prephenate Aminotransferase Directs Plant Phenylalanine Biosynthesis via Arogenate. Nature Chem. Biol., DOI:10.1038/nchembio.485
- Maeda H., Shasany A.K., Schnepp J., Orlova1 I., Taguchi G., Cooper B.R., Rhodes D., Pichersky E. and Dudareva N. (2010) RNAi Suppression of Arogenate Dehydratase1 Reveals That Phenylalanine Is Synthesized Predominantly via the Arogenate Pathway in Petunia Petals. Plant Cell 22, 832-849 *Described as a Research Highlight in Nature Chem. Biol. 6, 310
- Song W., Maeda H., and DellaPenna D. (2010) Mutations of the ER to plastid lipid transporters (TGD1, 2, 3 and 4) and the ER oleate desaturase (FAD2) suppress the low temperature-induced phenotype of Arabidopsis tocopherol deficient mutant vte2. Plant J. 62, 1004-1018
- Orlova I., Nagegowda D.A., Kish C.M., Gutensohn M., Maeda H., Varbanova M., Fridman E., Yamaguchi S., Hanada A., Kamiya Y., Krichevsky A., Citovsky V., Pichersky E., and Dudareva N. (2009) The Small Subunit Snapdragon Geranyl Diphosphate Synthase Modifies the Chain Length Specificity of Tobacco Geranylgeranyl Diphosphate Synthase in Planta. Plant Cell 21, 4002-4017
- Maeda H., Sage T.L., Isaac G.., Welti R., and DellaPenna D. (2008) Tocopherols Modulate Extra-Plastidic Polyunsaturated Fatty Acid Metabolism in Arabidopsis at Low Temperature. Plant Cell 20, 452-470 *Described in the Featured Article of the issue Plant Cell 20, 246
- Maeda H. and DellaPenna D. (2007) Tocopherol Functions in Photosynthetic Organisms. Curr. Opin. Plant Biol. 10, 260-265
- Maeda H., Song W., Sage T.L. and DellaPenna D. (2007) Tocopherols Play a Limited Role in Photoprotection but a Crucial Role in Chilling Adaptation in Arabidopsis Leaves. In Current Advances in the Biochemistry and Cell Biology of Plant Lipids, C. Benning and J. Ohlrogge, eds (Aardvark Global Publishing Company, LLC, Salt Lake City, UT), pp. 112-115 PDF download (4.5 MB)
- Maeda H., Song W., Sage T.L. and DellaPenna D. (2006) Tocopherols Play a Crucial Role in Low Temperature Adaptation and Phloem Loading in Arabidopsis. Plant Cell 18, 2710-2732 *Highlighted on the Cover of the issue.
- Sakuragi Y., Maeda H., DellaPenna D. and Bryant D.A. (2006) α-Tocopherol Plays a Role in Photosynthesis and Macronutrient Homeostasis of the Cyanobacterium Synechocystis sp. PCC 6803 That is Independent of its Antioxidant Function. Plant Physiol. 141, 508-521
- Maeda H., Sakuragi Y., Bryant D.A., and DellaPenna D. (2005) Tocopherols Protect Synechocystis sp. Strain PCC 6803 from Lipid Peroxidation. Plant Physiol. 138, 1422-1435
- Cheng Z., Sattler S., Maeda H., Sakuragi Y., Bryant D.A., and DellaPenna D. (2003) Highly Divergent Methyltransferases Catalyze a Conserved Reaction in Tocopherol and Plastoquinone Synthesis in Cyanobacteria and Photosynthetic Eukaryotes. Plant Cell 15, 2343-2356
- Okazawa A., Maeda H., Fukusaki E., Katakura Y., and Kobayashi A. (2000) In Vitro Selection of Hematoporphyrin Binding DNA Aptamers. Bioorg. Med. Chem. Lett. 10, 2653-2656
- Fukusaki E., Kato T., Maeda H., Kawazoe N., Ito Y., Okazawa A., Kajiyama S. and Kobayashi A. (2000) DNA Aptamers that Bind to Chitin. Bioorg. Med. Chem. Lett. 10, 423-425
|